
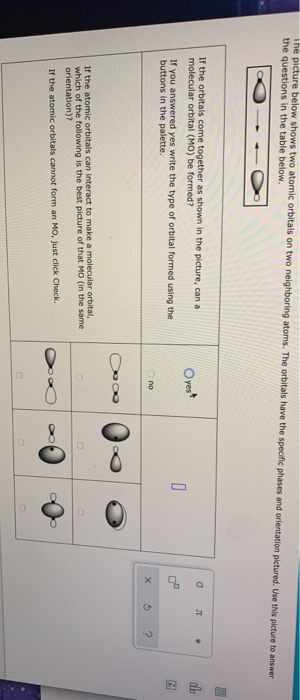
When p orbitals overlap end to end, they create σ and σ* orbitals.

When regions of opposite phase overlap, the destructive wave interference decreases electron density and creates nodes. When orbital lobes of the same phase overlap, constructive wave interference increases the electron density. In p orbitals, the wave function gives rise to two lobes with opposite phases. Electrons fill the lower-energy bonding orbital before the higher-energy antibonding orbital. Hence, these orbitals are called antibonding orbitals. The attractive force between the nuclei and these electrons pulls the two nuclei apart. Electrons in the σ s* orbitals are located well away from the region between the two nuclei. Adding electrons to these orbitals creates a force that holds the two nuclei together, so these orbitals are called bonding orbitals. Electrons in a σs orbital are attracted by both nuclei at the same time and are more stable (of lower energy) than they would be in the isolated atoms. The asterisk signifies that the orbital is an antibonding orbital. The out-of-phase addition (or subtracting the wave functions) produces a higher energy σ s* molecular orbital (read as "sigma-s-star"), in which there is a node between the nuclei. The in-phase combination produces a lower energy σ s molecular orbital (read as "sigma-s") in which most of the electron density is directly between the nuclei. There are two types of molecular orbitals that can form from the overlap of two atomic s orbitals on adjacent atoms. Bonding and Antibonding Molecular Orbitals In orbitals, the waves can combine with in-phase waves producing regions with a higher probability of electron density and out-of-phase waves producing nodes, or regions of no electron density. Combining waves can lead to constructive or destructive interference. Quantum mechanics describes molecular orbitals as combinations of atomic orbital wave functions. The mathematical process of combining atomic orbitals to generate molecular orbitals is called the linear combination of atomic orbitals (LCAO).

COMBINING ATOMIC ORBITALS FULL
Like an atomic orbital, a molecular orbital is full when it contains two electrons with opposite spin. The region of space in which a valence electron in a molecule is likely to be found is called a molecular orbital ( Ψ 2). Just like electrons around isolated atoms, electrons around atoms in molecules are limited to discrete (quantized) energies. Quantum mechanics describes the behavior of an electron in a molecule by a wave function, Ψ, analogous to the behavior in an atom. Molecular orbital theory describes the distribution of electrons in molecules in the same way as the distribution of electrons in atoms is described using atomic orbitals. So, two 2 s orbitals can overlap, but a 2 s orbital generally has negligible overlap with a 1 s or 2 p orbital. Orbitals can overlap if their energies are similar and their symmetries match. The π bonding orbitals are typically equal in energy, or degenerate, as are the π antibonding orbitals. The orientation of the three different p orbitals means that typically, one pair overlaps end-to-end and the other two pairs overlap sideways. Here, the electron density is concentrated on opposite sides of the internuclear axis. Sideways overlap, such as the side-on overlap of two p orbitals, results in π molecular orbitals. The σ orbital electron density is centered about the internuclear axis. Head-on combination of atomic orbitals along the internuclear axis, such as the overlap between two s orbitals or two end-to-end p orbitals, results in σ molecular orbitals. Molecular orbitals are classified by the way the atomic orbitals overlap. This antibonding molecular orbital is higher in energy than the atomic orbitals and is marked with a star or asterisk. This bonding molecular orbital is lower in energy than either of the original atomic orbitals.ĭestructive interference between out-of-phase atomic orbitals corresponds to lower electron density in a nodal plane between the nuclei, making the molecule less stable. Waves can interact either constructively or destructively.Ĭonstructive interference between in-phase atomic orbitals corresponds to greater electron density between the positively charged nuclei, making the molecule more stable. These functions are estimated by a mathematical process called the linear combination of atomic orbitals. Like atomic orbitals, molecular orbitals are wave functions describing where electrons are likely to be. Molecular orbital theory describes the distribution of electrons throughout a molecule rather than localizing them to specific bonds between atoms.


 0 kommentar(er)
0 kommentar(er)
